
So there's my one proton in the nucleus, and we're talking about a neutral hydrogen atom, so there's one electron. We know the atomic number of hydrogen is one, so there's one proton in the nucleus. So let's go ahead and draw an atom of hydrogen. In a neutral atom, the number of protons is equal to the number of electrons, because in a neutral atom there's no overall charge and the positive charges of the protons completely balance with the negative charges of the electrons. So it's right here, so there's one proton in the nucleus of a hydrogen atom.

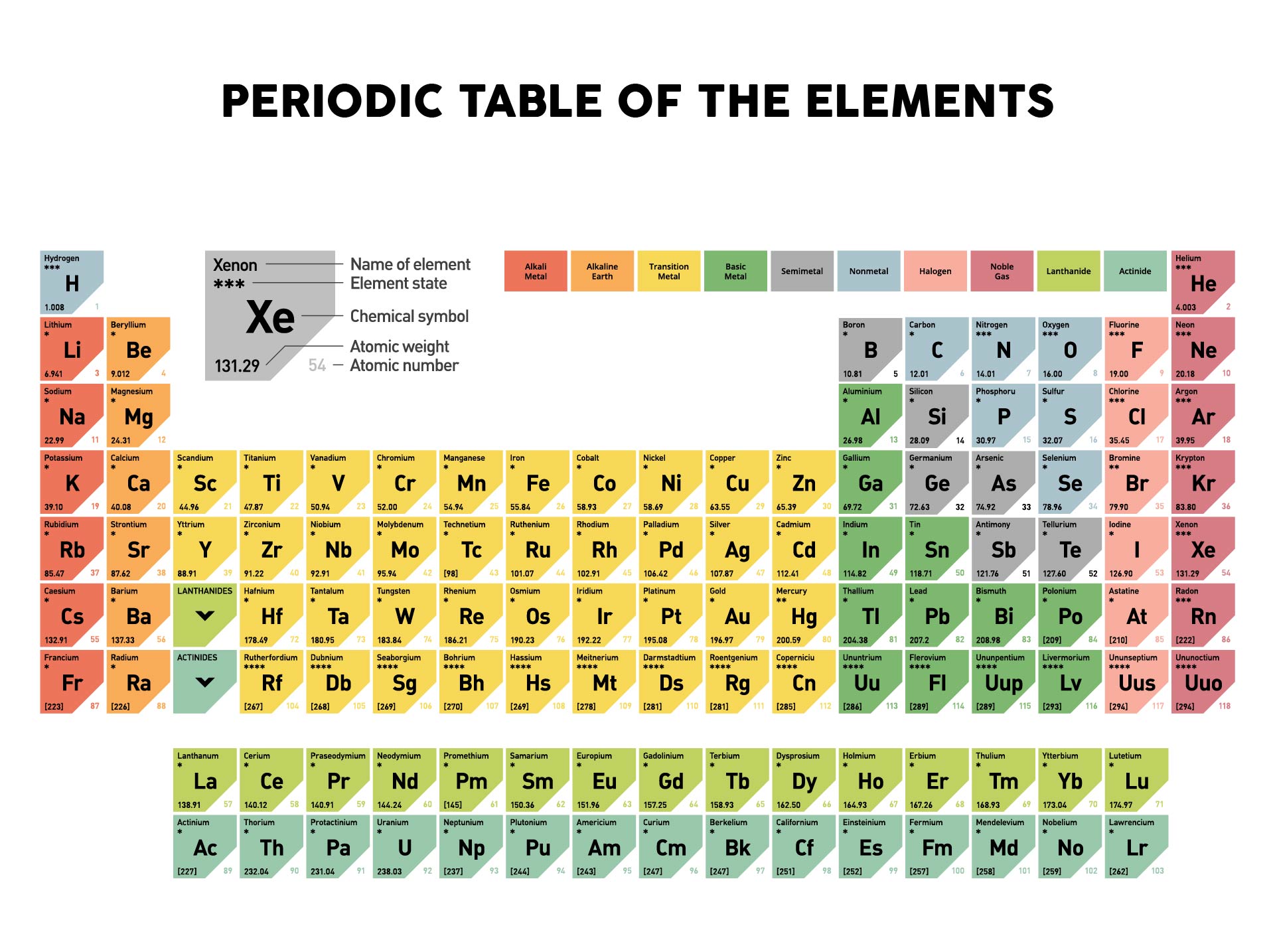
So for hydrogen, hydrogen's atomic number is one. So we're going to talk about hydrogen in this video. And you can find the atomic number on the periodic table. So the atomic number is symbolized by Z and it refers to the number of protons in a nucleus. There are ways it can happen, but they are not the normal course of events. So you do not usually have one isotope changing into another isotope of the same element. So, it is not possible to give you a general answer other than to say that the isotopes of an element form in one or more of the above ways, but each isotope of an element may or may not form in a similar way as other isotopes.Įxcept as listed above, an atom that is not radioactive never changes its number of neutrons. There are a few other ways, but they are not all that significant.Įach isotope (more properly called a nuclide) has its own way(s) in which it is formed.
Being changed from one type of atom to another by high energy nuclear reactions, such as having a neutron slam into the nucleus at an extremely high velocity.
The universe began with simple elements like hydrogen and helium that under high heat and pressure were forced together binding their protons and neutrons with a strong nuclear force.With the exception of the hydrogen and helium that were formed shortly after the Big Bang event, elements mainly form in the following ways:Ĥ. Under these conditions it is possible to undergo nuclear fusion, adding nucleuses together to increase the number of protons and neutrons. This is a natural phenomenon, when the universe first began stars were areas of high gravity, heat and force. This is because a proton is added to the nucleus each time. This means as we progress along a period (from side to side) or down a group (up and down) the atoms get progressively larger usually by a unit of one. And as you progress down the periodic table the atomic numbers also increase. A key trend in the periodic table is its ascending number of protons from left to right, going across the periods. This difference in the number of protons means not only are they different elements but they are at different places in the periodic table. For example, Carbon has 6 protons and Chlorine has 17. This is what determines what element it is.

has one proton in the nucleus and would have a mass of 1.672622x10-27 Kg.Įvery element has a different number of protons in the nucleus. If we looked at the atomic mass of Beryllium considering the real mass of a proton (1.672622x10-27 Kg) the actual atomic mass would be 9.012182 x10 -27 Kg when considering the mass of protons in Kg. mass of 1.įor example the atomic number of Beryllium is 4 as it has 4 protons in the nucleus each worth a mass unit of 1. As we say that a proton has a mass of 1 for every proton we have in the nucleus this corresponds to an increase in a. The atomic number of an element links back to the mass of a proton. Dimitri Mendeleev initially began by structuring the periodic table based on the different elements' chemical properties, much as it still is today but a key concept underpinning the periodic table is Atomic Number. The periodic table as laid out by Dimitri Mendeleev, the founding father of the periodic table is based on a key concept that everything relates to the mass of the atom.


 0 kommentar(er)
0 kommentar(er)
